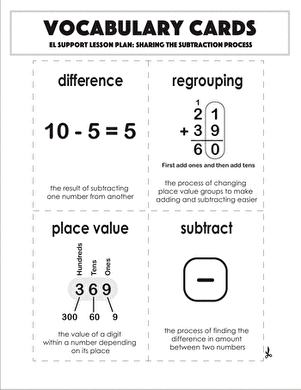
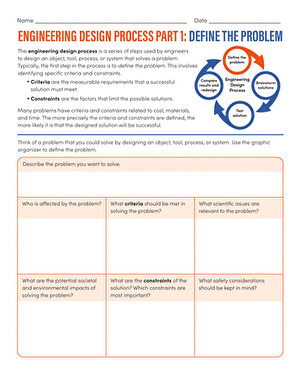
Activity
The Process of Diffusion
Grade Level: 9th - 12th; Type: Life Science
Objective:
To investigate the process of diffusion.
Research Questions:
- What is the difference between osmosis and diffusion?
- How is diffusion regulated?
You can see beautiful colors everywhere you look! There is a lot of science behind the colors that you see. Color is the property that our eyes visually perceive and is derived from the spectrum of light. White light is a blend of all the spectrum colors which include red, orange, yellow, green, blue, indigo, and violet. Individual colors of white light can be seen by using a prism which separates the colors and, in turn, white light can be seen when the spectrum colors are combined in such a fashion as a spectrum spinner.
Materials:
- 1 plastic baggie
- Corn starch
- Iodine
- Tap water
- Stopwatch
- Twist tie or rubber band
- Clear drinking glass
Experimental Procedure:
- Place ½ cup of water and 1 tsp of corn starch into the baggie.
- Seal the baggie and also place the twist tie or rubber band on it as an extra security measure.
- Fill your drinking glass half full with tap water.
- Place 8 - 12 drops of iodine in the water.
- Place the baggie with the corn starch solution in the glass with the iodine solution. Make sure the corn starch solution is submerged in the iodine solution.
- Wait 15-20 minutes.
- Remove the baggie from the glass and place it on a paper towel. Do not unseal the baggie.
- What do you notice about the color of the two solutions?
- Complete Table 1.
- Which solution passed through the baggie? Which solution did not pass through the baggie?
- Explain why one solution could and one could not pass through the baggie.
- Hypothesize what the results would be if the two solutions were to switch their placement (iodine solution in the baggie and corn starch solution in the glass). If time permits feel free to test your hypothesis.
Beginning color |
Ending color |
|
Corn starch solution |
||
Iodine solution |
Terms/Concepts: Solution concentration; Cell membrane; Selective permeability; Diffusion; Osmosis
References:
http://www.biologycorner.com/bio1/diffusion.html
http://hyperphysics.phy-astr.gsu.edu/hbase/kinetic/diffus.html
Education.com provides the Science Fair Project Ideas for informational purposes only. Education.com does not make any guarantee or representation regarding the Science Fair Project Ideas and is not responsible or liable for any loss or damage, directly or indirectly, caused by your use of such information. By accessing the Science Fair Project Ideas, you waive and renounce any claims against Education.com that arise thereof. In addition, your access to Education.com's website and Science Fair Project Ideas is covered by Education.com's Privacy Policy and site Terms of Use, which include limitations on Education.com's liability.
Warning is hereby given that not all Project Ideas are appropriate for all individuals or in all circumstances. Implementation of any Science Project Idea should be undertaken only in appropriate settings and with appropriate parental or other supervision. Reading and following the safety precautions of all materials used in a project is the sole responsibility of each individual. For further information, consult your state's handbook of Science Safety.