Activity
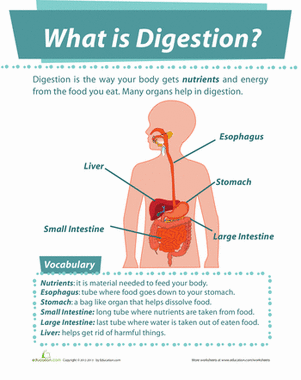
What Happens When We Mix Acid with Base Solutions?
Grade Level: 6th - 8th; Type: Chemistry
Objective:
Discover whether mixing solutions of equal distances from neutral pH (7) will create a solution close to pH 7. If not, what happens to the pH level of these solutions once they are mixed?
Research Questions:
- How can you definite an acid? A base?
- What are some other acids and bases you found around the house?
- Why is pH important to life?
pH is the way to measure how acidic or basic a solution is. The scale ranges from 0-14, with 0 being the most acidic and 14 being the most basic. 7 is neutral- it is neither basic nor acidic and stands for distilled water. An example of an acidic solution would be any kind of citrus juice and an example of a basic solution would be baking soda or soap.
Materials:
- Milk
- Egg whites
- Rainwater
- Baking Soda
- Tums Antacid
- Soda
- Ammonia
- Vinegar
- pH paper
- A few glass beakers or containers
- A medicine dropper
- Water
Experimental Procedure:
- Before mixing anything together, dip a piece of pH paper inside each solution to check the pH of each.
- Take medicine dropper and draw up 10 drops of milk and 10 drops of liquidy egg whites. Be sure to rinse the dropper in between solutions if you are using only one to prevent contamination.
- Mix these solutions together well.
- Take a piece of pH paper and dip it in the mixture. Record the color.
- Now take the medicine dropper and repeat steps 1-3 but with these combinations: Rainwater & baking soda; Tums Antacid & soda; Ammonia & vinegar
- Access whether any of the above combinations create a mixture of a pH of 7 of which is the closest to it. If it did does that necessarily mean it is water?
Suggested Chart
|
Separated |
Color Shown on pH Paper |
Equivalent pH Value |
|
Milk |
|
|
|
Egg Whites
|
|
|
|
Rainwater
|
|
|
|
Baking Soda |
|
|
|
Tums
|
|
|
|
Soda
|
|
|
|
Ammonia
|
|
|
|
Vinegar
|
|
|
|
Mixed |
Color Shown on pH Paper |
Equivalent pH Value |
|
Milk + Egg Whites |
|
|
|
Rainwater + Baking Soda
|
|
|
|
Tums + Soda |
|
|
|
Ammonia & Vinegar |
|
|
Terms/Concepts: pH scale; acidity and basicity
References:
- http://www.elmhurst.edu/~chm/vchembook/184ph.html
- http://www.visionlearning.com/library/module_viewer.php?mid=58
- http://library.thinkquest.org/3659/acidbase/ph.html
- Understanding Acids and Bases (Chemicals in Action) by Chris Oxlade
Education.com provides the Science Fair Project Ideas for informational purposes only. Education.com does not make any guarantee or representation regarding the Science Fair Project Ideas and is not responsible or liable for any loss or damage, directly or indirectly, caused by your use of such information. By accessing the Science Fair Project Ideas, you waive and renounce any claims against Education.com that arise thereof. In addition, your access to Education.com's website and Science Fair Project Ideas is covered by Education.com's Privacy Policy and site Terms of Use, which include limitations on Education.com's liability.
Warning is hereby given that not all Project Ideas are appropriate for all individuals or in all circumstances. Implementation of any Science Project Idea should be undertaken only in appropriate settings and with appropriate parental or other supervision. Reading and following the safety precautions of all materials used in a project is the sole responsibility of each individual. For further information, consult your state's handbook of Science Safety.